There is an idea that floats around homeopathy called “water memory:” that water can “remember” substances dissolved in it (or antibodies) even after extreme dilution. Put another way, it’s the idea that minuscule aspects of something in the water are carried by the entire body of water sufficiently to matter for medicinal purposes.
The concept was published in a reputable scientific journal in 1988 and then debunked over following decades. However, due to the publishing whiplash and the great marketing ideas that use it to justify various products, it has persisted. (There is a decent history of it here.) But don’t forget: you can just dilute something out of meaningful existence.
In my last semester of grad school (two years ago! yikes!), I took a seminar on stable isotope techniques (taught by Todd Dawson of the Center for Stable Isotope Biogeochemistry), and I share this because stable isotopes in hydrology felt as close as you can scientifically get to the idea of water memory. It felt like magical science. So let me explain!
Let’s start with what an isotope is. All of the elements in the periodic table have default atomic mass values. Those are the little numbers in the bottom right corners of each element in this graphic:
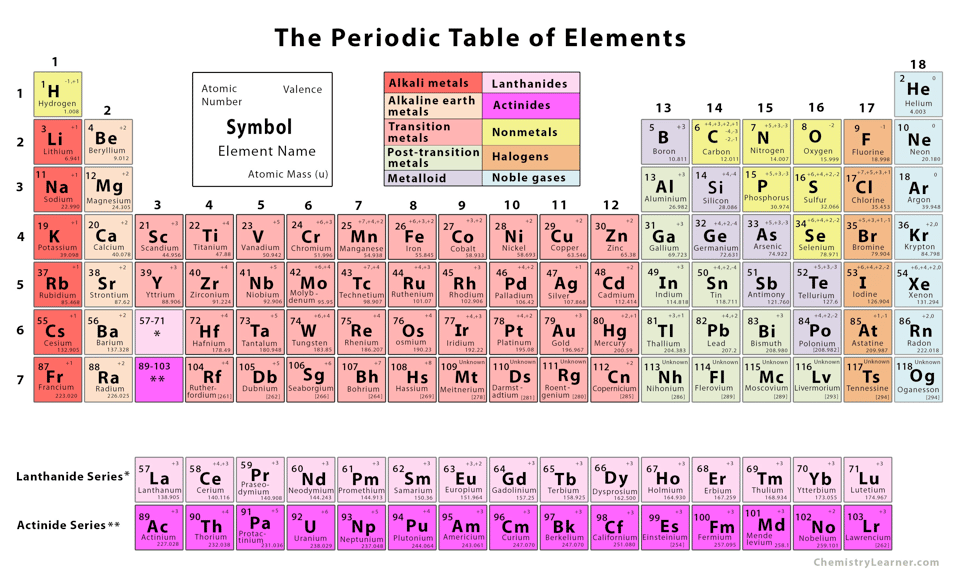
These weights are the combination of the mass of the protons and neutrons (and electrons, but those are relatively tiny). You’ll notice the masses tend to be nearly integers. If an atom changes its number of protons, it becomes a different element. If it changes the number of electrons, it gets a different charge, and these things matter a lot. However, if an atom changes its number of neutrons… not a ton happens. (Forgive me, chemists!) So atoms can gain or lose neutrons, changing their atomic masses.
The variety in the number of neutrons an element can have are called its isotopes. Carbon, for instance, has a lot of isotopes: it has 6 protons, an it can have anywhere between 2 neutrons (Carbon-8) and 16 neutrons (Carbon-22). However, isotopes come in two flavors: stable and unstable. Unstable isotopes are also called radioisotopes. For reasons honestly beyond me (hey, I haven’t taken chemistry in 15 years), elements are only “happy” in a couple isotope configurations. Otherwise, if you slap on more neutrons, they’ll get tossed off via radioactive decay. Carbon only has two stable isotopes: Carbon-12 (the “default”) and Carbon-13 (carbon… with an extra neutron!). This stability means that Carbon-13 moves through the universe much like Carbon-12 does, albeit carrying around an bit of extra weight. It is not inclined to throw away the bonus neutron the way radioactive stuff does. Some elements have zero stable isotopes.
In the class I took, the entire focus was the stable isotopes, particularly for use in biological, ecological, and environmental sciences. It is the stable isotopes that are out in the environment, rather than the wackier radioisotopes that generally require fancy labs or astronomical processes to manufacture. Often, these isotopes are all hanging out together naturally in mixtures. Now, crucially, when elements go through some kind of process that interacts with them at an elemental/molecular level, those processes often sort the isotopes by weight. This is some of the key magic in stable isotope techniques, and it is called “fractionation.”
Because this is a newsletter mostly about water, I’ll focus on that: water has oxygen and hydrogen elements and thus their isotopes, too. When water evaporates, it fractionates: the “heavy water” (with Hydrogen-2 or Oxygen-17 or -18) usually stays behind in the liquid water, and the lighter isotopes evaporate into water vapor. The reverse happens when it condenses: the heavier elements will become water first.
Fractionation happens in phase changes between solid, liquid, and gas. It happens in chemical reactions, where typically the lighter isotopes are preferentially used-up. It happens as compounds are metabolized by organisms. It happens when substances diffuse through various media. You’re fractionating right now!!! The big idea behind stable isotope study is that if we have a good model of how the fractionation happens, we can use the isotopic composition of a sample to tell us what it has been through.
A bit of water falls on a mountaintop, and as it flows down a stream, some of it evaporates—making the water that remains on earth get a bit “heavier.” Then perhaps a beaver drinks it up and perspires, preferentially sweating out the light isotopes. Then a biologist comes along and plucks a hair from the beaver and compares it to the default isotope of the region where the animal was found. If we have decent models of how evaporation fractionates, and how beavers sweat (do they???), we can see how those effects aggregate together to the water in the beaver hair. The water carries an isotopic fingerprint of the processes that have touched it. The water remembers. And then we can ask some cool questions about that, like “did the beaver travel from far away to where we found it?” If so, its hair’s isotopic composition is likely to be very different than the norm of where it was found.
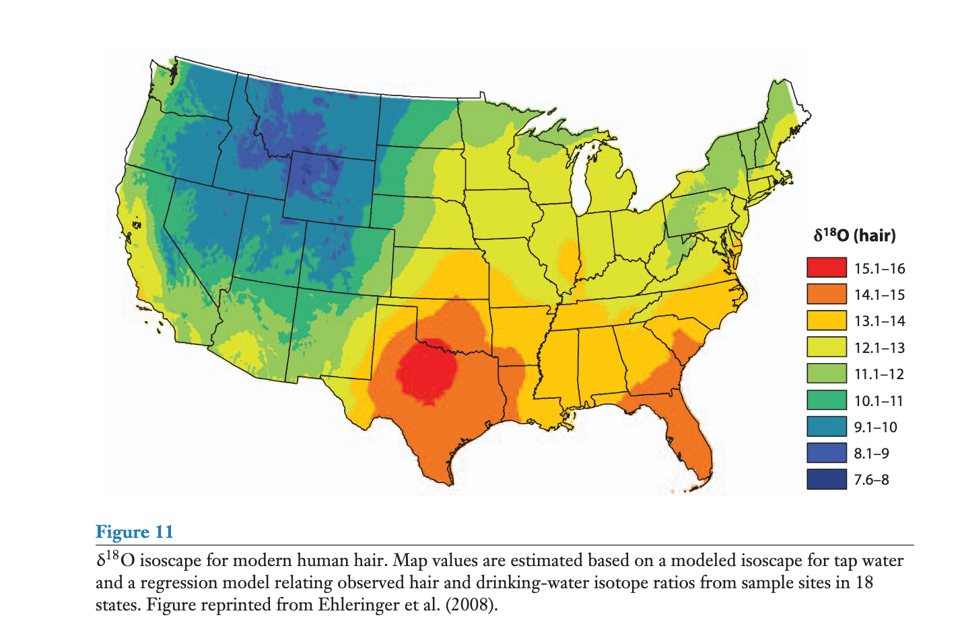
These regional norms are crucial, and are now a big part of the science, called “isoscapes” (isotopic… landscapes…). People have worked really hard to make good baseline understanding of how common environmental elements—hydrogen, oxygen, carbon, nitrogen… and strontium, actually—have stable isotopes that vary spatially and consistently. This is particularly so for the water isotopes, because we are on the blue planet after all. Because of different source waters, rainfall varieties, and evaporation rates, water isotopes vary fairly consistently across large spatial scales. Look at the map above, for signatures of Oxygen-18 in human hair. The isotope notation requires some learning, but imagine this: if someone finds a hair with a 15.5-valued d18O hair in central Montana, we could assume they’ve actually spent most of their time in central Texas. By combining isotopes of a few different elements (and some crafty assumptions), you can pinpoint where a sample has been.
We can do these same kinds of forensics with water in the landscape, by sampling the isotopes of a vial to figure out where it came from, or how long it has been there. This is especially useful for groundwater, which is more difficult to “see” move. It requires access to the right mass spectrometer and a conceptual model of what has caused fractionation, or a plausible story of how the water has moved through the environment. And the belief that the water remembers where it came from… isotopically, of course.
I’m not directly working on water isotopes right now, but I’m curious if incorporating them into my project would answer some key research questions. I might even try to go to IsoCamp next summer.
Remembering,
Lukas
p.s… for those interested in the “natural fiber gusseted pants” quest from Gnamma #97, Nate and I are trying the affordable and 100% cotton bike pants from the ever-lovable Blue Lug (Japan). Ask me about them later… I need to pick up my pair.
You just read issue #98 of Gnamma. You can also browse the full archives of this newsletter.