
Why The Magic?
One of the most surprising recent trends I've noticed in science news is the explosion of stories about magic mushrooms. Of course, this is old news for people across Latin America who have consumed the fungi in rituals for thousands of years. It was only in 1957 that many Americans became aware of psilocybin, the active compound in magic mushrooms. In an article for Life magazine a J.P. Morgan banker named Robert Gordon Wasson recounted how a Mexican shaman provided him with the legendary substance. "For the first time the word ecstasy took on real meaning," Wasson reported.
To Wasson's regret, the article brought a flood of hippies to Mexico seeking to copy his experience. The counterculture popularity of the mushrooms led the U.S. government in 1971 to designated them a Schedule 1 controlled substance. A hidden culture grew around the illegal fungi like a subterranean web of mycelia.
Today, over half a century later, they're still officially illegal for the most part. But in recent years some scientists have begun carrying out studies to understand how exactly they produce ecstasy in the human brain. Psychiatrists are investigating them as a way to treat patients for with depression, PTSD, and other conditions. (A few months ago, I had a fascinating conversation at the Crosscut Festival in Seattle with two psychiatrists in the midst of this work. You can listen to it here.)
Those lines of research are interesting and important, but here at "Friday's Elk," I like to spin the anthropocentric telescope around and look through the other end. Let's remember that these fungi do not make their psilocybin for us. So why are some mushrooms magic?

There must be a good reason. Psilocybin isn't waste that a mushroom casts off as it makes other things. It has to put a lot of effort into manufacturing the molecule. The effort starts even before the production of psilocybin itself. First, the mushroom has to make several enzymes. One of those enzymes alters a common amino acid called tryptophan. Then another enzyme takes over and makes another change. Then another, then another. Step by step, the enzymes transform a common amino acid into a very uncommon molecule.
One step towards understanding why mushrooms would go to all this effort to make psilocybin is to trace its evolution. To that end, a team of American, Mexican, and British scientists have reconstructed a deep history of psilocybin, which they published this week in the journal Proceedings of the National Academy of Sciences.
The history of psilocybin is one chapter in the long success story of fungi. Fossils suggest that fungi first evolved a billion years ago. They set themselves apart from other life forms by evolving enzymes that they spewed out to break down organic material around them. Then they soaked up the decomposed matter as food. Over the next billion years, fungi adapted to a staggering number of ways to make a living. That versatility has helped them evolve into an estimated 2 to 3 million species.
Fungi probably first thrived in rivers and ponds, feeding on algae. Then they moved on land, probably following algae that evolved into plants. Some of the early fungi broke down dead plant matter, while others attacked live ones as parasites. And still other fungi formed partnerships with the plants, coating their leaves with a protective shield and supplying nutrients to their roots.
They took advantage of animals, too. Some managed to live harmlessly on animal bodies. Some formed partnerships--fungi are among the microbes that cows keep in their rumen to help break down grass, for example. Some fungi evolved to thrive in the dung animals left behind.
We humans are a fungal wonderland of our own. While some fungi cause lethal infections, many live quietly in our guts and mouths, using their enzymes to suck up loose food or attack bacteria. Our fungi may even keep us healthy, repairing our skin and carrying out other useful jobs.
As fungi diversified over the past billion years, they diversified their portfolio of enzymes, which they used not just to break down food, but to build new compounds. Fungi found many jobs for their new molecules, such as warding off bacteria. One of the compounds they make for this task is penicillin.
Penicillin offers an important lesson about how to think about magic mushrooms. We humans found a valuable use for penicillin, but fungi didn't evolve a way to make it so that we could cure our own infections. Penicillin benefited fungi first. The same must be true for psilocybin.
Psilocybin got its name from Psilocybe, the genus of mushrooms in which the molecule was first identified. There is no one "magic mushroom"--mycologists have found about 165 Psilocybe species, and there may well be more. Making matters more complex, mycologists have also found some species outside of the Psilocybe genus that make psilocybin, too.
To sort out this evolutionary mess, the authors of the new study visited museum collections and snipped samples of 71 species of Psilocybe. Then they sequenced DNA from the samples, and used the mutations in each species to draw a family tree, tracing the species back along their branches to their common ancestor. By comparing the mutations along each branch of the tree, the scientists were able to estimate when that common ancestor lived.
That common ancestor lived about 67 million years ago according to the new study. That was around the time an asteroid smashed into the planet and caused a mass extinction. The impact is best known for wiping out all dinosaurs except for birds. But it also destroyed entire forests by blocking the sun and making the atmosphere toxic.
This was a good time to be a fungus that fed on plants. It may be no coincidence that paleontologists have found a "fungal spike" in layers of rock that formed just after the impact. The deepest branches of the Psilocybe evolutionary tree include species that today grow on dying plant matter. That may be a sign that this is how the whole lineage of magic mushrooms got its start. I like to imagine them sprouting in dark, dying forests littered with the corpses of tyrannosaurs.
The researchers then traced the history of the enzymes that produce psilocybin. There is no one way to make the compound, it turns out. Different species used different versions of the same enzymes. The order in which the genes for the enzymes are arranged in the mushroom DNA is also different among species.
The authors of the new study speculate that when Psilocybe first evolved to feed on dying plants, they did not yet make psilocybin as we know it. Only millions of years later did two Psilocybe lineages evolve new ways of making a living. They started growing in the soil or in the dung of mammals. And it was only after these shifts that the two lineages independently evolved psilocybin.
The new study also helps makes sense of how mushrooms outside the Psilocybe lineage ended up making psilocybin too. Starting about 40 million years ago, the genes for psilocybin jumped from Psilocybe mushrooms to other species. This process, called horizontal gene transfer, has happened a lot among fungi. The tendrils of two species may make contact with each other, causing their cells to fuse and giving DNA from one species the chance to move into the other. In other cases, fungi just slurp up DNA along with their regular meals. If the DNA is useful to the species that receive it, it will pass it down through the generations.
What's striking about the horizontal transfer of psilocybin genes is that they have made the jump together a number of times to different species. And after the jump, they've all endured together in their new hosts for millions of years. That endurance suggests, yet again, that for some fungi there's something really good about being able to make psilocybin.
A few months ago, two experts on psilocybin at the Ohio State University--Matthew Meyer and Jason Slot--published a review of what that benefit might be. One of the most intriguing suggestions--which the authors of the new study are going to look into--is that the magic in magic mushrooms evolved as a weapon.
They used that weapon, the scientists suggest, against slugs and snails. These gastropods are, Meyer and Slot write, "voracious fungivores." Perhaps psilocybin evolved as way for mushrooms to ward off these hungry enemies.
But Meyer and Slot also raise an even weirder possibility--one that I would have loved to include in Parasite Rex, my book on how surprisingly sophisticated parasites are.
Some parasites control their hosts, forcing them to do things for their own benefit. This includes fungi. Cordyceps, which offered inspiration for the HBO show "The Last of Us," infects ants and forces them to climb up plants. The fungi then push a stalk out of their host's body and shower spores down on the ants below. Other fungi play a similar game of puppet master with flies.
Imagine then, that psilocybin evolved as a way to control a host's behavior.
Let's not forget the thing that got people interested in psilocybin in the first place: its ability to alter the human nervous system. It seems to do so thanks to its molecular similarity to serotonin, one of the key neurotransmitters in our bodies. We are not unique in making serotonin: other animals do too, including snails and slugs.
So imagine a snail visits a pile of ground sloth dung back in the Miocene to enjoy a meal of Psilocybe. Its meal includes not just mushroom tissue, but a dose of psilocybin. The snail experiences a gastropod version of a shroom trip. The tripping snail wanders off, traveling a longer distance than it otherwise would with a clear mind. Inside its gut are hard fungal spores that it could not digest. The snail then releases the spores in its own droppings. Thus a mushroom might use snails to expand its range, allowing its species to endure for millions of years.
This is not a pitch for a new HBO series. It's a testable hypothesis. By exploring this idea and others like it, scientists may get a deeper understanding how psilocybin works, and how it might benefit us.
Ancient Genes and Modern Ailments
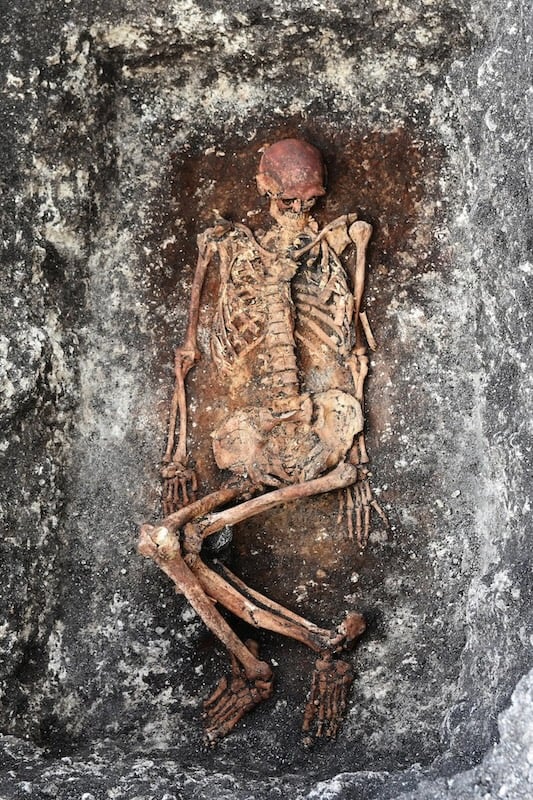
For my Origins column in The New York Times this week, I explore a gigantic study of over 1,000 genomes of people who lived in Europe and Asia from the end of the Ice Age 11,000 years ago through the Bronze Age to about 3,000 years ago. What makes this study especially noteworthy is that the researchers linked the genetic mutations of these long-lost people to medical records and DNA of living people. The result is a new insight into current-day diseases such as multiple sclerosis.
Long-term "Friday's Elk" readers will know I've been tracking ancient DNA research for a long time. Here, for example, is a 2016 profile I wrote of Eske Willerslev, the University of Copenhagen geneticist who went on to lead the new effort. There was a time when retrieving a single piece of ancient DNA was huge news. It's remarkable that scientists can now compare thousands of ancient genomes. I expect that as Willerslev team sequences more DNA and looks at more disorders, such as schizophrenia and Alzheimer's, I'll be reporting on them again in the future.
That's all for now.
Best,
Carl
You just read issue #165 of Friday's Elk. You can also browse the full archives of this newsletter.