Car Science: Blowing smoke
Hello! You're reading Car Science; please consider subscribing, it's free and helps me.

Hey,
This is also not the email with a long bit apologising for not having sent an email for ages that's about metamaterials. That's just taking me ages to write because it's about buckling metamaterials and I am a car guy with three braincells.
So: sorry about all those emails I didn't send, stuff's been really bad. Pretty irredeemably bleak, if I'm honest. Didn't really think we were, by which I mean I was, gonna make it to the sunlit uplands of the post-Barbie universe but Friday's looking suspiciously achievable at this rate.
Unrelenting horror aside, though, it is Wednesday and I should send something, so here we are. Time to talk about direct carbon capture systems for vehicle emissions.
Here's the thing: when vehicles burn hydrocarbon fuels in combustion engines there's CO2 released as an exhaust gas. There's no way around that, in chemical terms: you combust the hydrocarbon, you need oxygen around and that means as you break the chains of hydrogen and carbon and release the energy, you'll end up with carbon dioxide.
Unlike, say, diesel NOx emissions there's no way around it, either. Diesel or hydrogen can burn lower to reduce its NOx output but for petrol (or diesel or the filthiest fuel known to mankind) there's just no way around CO2 being generated.
Then once it's out the only way to get it back is by the painfully energy-intensive process of direct air carbon capture. Leaving aside the very real benefits of using plant life, the original carbon capture system, carbon capture is a messy and inexact technology that exists but is reliant on vast amounts of air being pumped through its system. So much so some scientific-diagrams-that-look-like-shitposts awhile back proposed mounting systems on long distance trains for passive airflow. I sure have written some articles.
So ideally, the best time to capture carbon is by leaving it in the ground. Once it's out, the second best time would be at the point it's exiting an engine as exhaust gas, so it never gets far into the air.
Great idea, right? Absolutely but this is Car Science so you already know there is a hitch. The problem is that the systems for it are just incredibly cumbersome. You're not talking about a catalytic converter, here, you're talking about the kind of thing it's looking potentially practical to fit to the vast smokestacks of freight ships.
That's a really positive move because any reduction in carbon emissions is crucial and shipping is a major contributor, as transport goes. But it's not a fix that would work on even a large road vehicle like an articulated lorry.
It's a shame because things like catalytic converters have been emissions miracles. They involve precious metals (platinum & platinum-group rare metals palladium and rhodium) but amazingly, the car industry could suddenly bandy around and roll them out when the alternative was not being able to sell cars. Something that seems to get conveniently forgotten when discussing the phase out of combustion, now.
But I digress. Back to the carbon dioxide capture. This week's issue of Cell Reports Physical Science has the cover picture that has gone the hardest of all-time on any scientific journal ever. Which honestly meant the contents were going to struggle to live up to it but there's a lot of interesting stuff in there, including a paper called Onboard capture and storage system using metal-organic frameworks for reduced carbon dioxide emissions from vehicles.
Vehicles, you say. I wonder if this is when Hazel reveals that by that they mean like gigantic mining equipment technically capable of movement or something?
No, this is actual vehicles. Heavy goods lorries, specifically, which have been the target for a bunch of technologies whether that's experimenting with trying to package as much hydrogen onboard as possible or fitting systems like this, since a large truck has a lot more possibilities for fitting abatement systems on than, say, my 1993 Renault Twingo does.
Even so, like I said, these systems have barely started being fitted to big ships. So working out a way to fit them to a freight truck is clever and extremely necessary, since the current abatement methods for trucking are very limited. About the best option is switching to HVO100 biofuel, which has a 4% tailpipe carbon emissions reduction and the advantage of not coming from fossil fuel, so so long as the oil comes from organic waste it's a significant improvement.
Actually stopping that stuff getting out, though? Now we're talking.
The system that's been developed works on tractor-trailer trucks (so, ones with a standalone front end you hitch the lorry to) with a payload of 25,600kg. That's the most common sort of heavy haulage vehicle and accounts for 75% of all heavy duty truck CO2 emissions, so it's well worth concentrating on. All the calculations are based on a turbocharged Volvo D13 diesel engine, again because that's the most commonly used on these type of vehicles. (90% of the heavy duty fleet is turbocharged diesel)
Here's where it gets pretty clever. So here's a diagram of where this system sits:
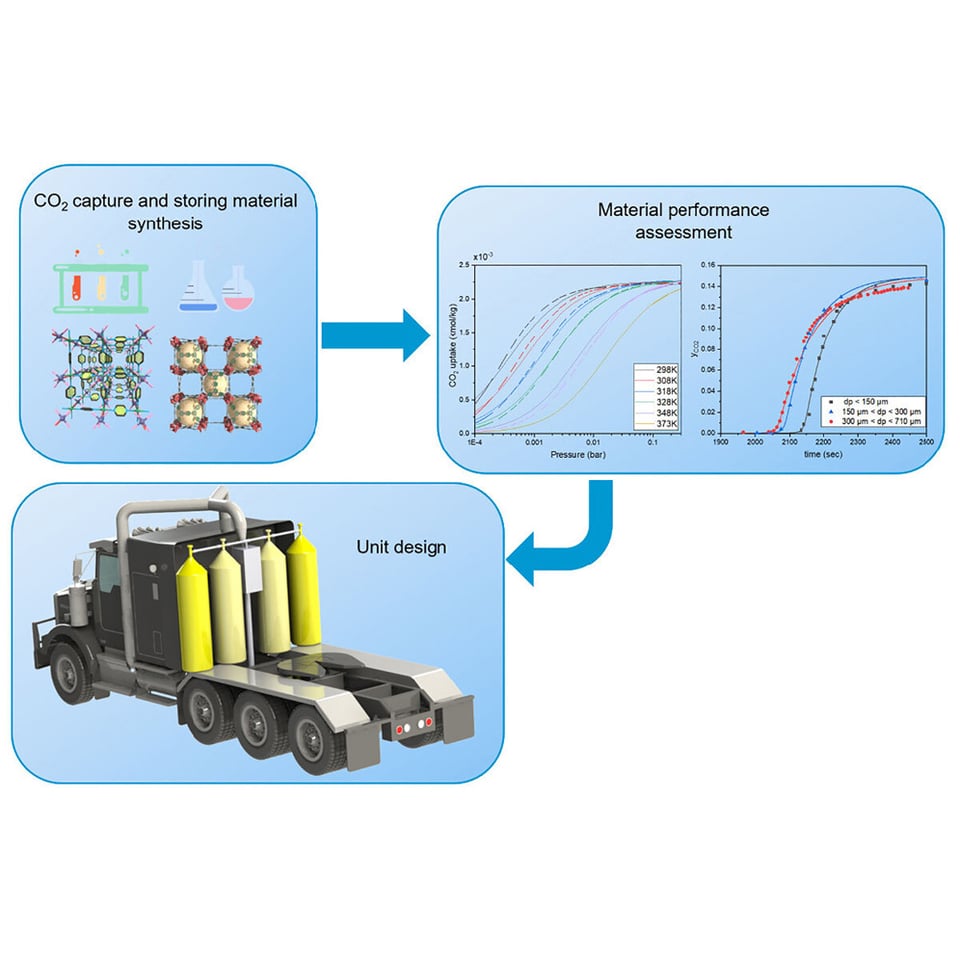
There's a whole bunch of problems with installing a system like this. The first is that CO2 storage is just really annoying; its relatively easy to capture as a gas but then you need a large volume to store it in. If you capture it and compress it as a liquid then that's much more efficient in space terms but horrifically energy consumptive and would need to draw significant power away from the engine to get it done.
That's obviously not going to be useful when the engine's meant to be hauling the stuff on the truck. So that's where the metal-organic framework comes in. KAUST-7 is a MOF that was developed at King Abdullah University of Science and Technology in Thuwal, Saudi Arabia, in 2016.
WARNING: SCIENCE BIT (SCROLL FOR END)
MOFs are - for the purpose of 99.9% of the ways we talk about them on Car Science - basically various sorts of catalytic foam. Sometimes they're sorbents, which is to say they can either absorb or adsorb things. In this instance the Al-soc-MOF-1 structure is being used because it's got a lot of porous surface area which CO2 can adhere to, which is adsorption - grabbing something by creating an external film of it, rather than being able to absorb it into the inside of something else.
Anyway, so KAUST-7 was originally developed to remove harmful propane from propylene and it uses niobium and nickel-based specific chemistry (NbOFFIVE-1-Ni if you're err, playing along in the lab) to adsorb propylene, meaning propane can be... siphoned is not the technical word here but let's say siphoned off without having to undergo the usual, energy intensive separation process for the two.
Turns out it can also be used as a CO2 adsorber, when the KAUST-7 is put into the Al-soc-MOF-1 crystal structure. Sorry, that probably got excessively chemically. I've been spending a lot of time on my own with YouTube and somehow managed to avoid being turned into a frothing conspiracy theorist but I AM super interested in the Czochralski process.
NOW RELATIVELY NORMAL AGAIN BY THE STANDARDS OF THIS NEWSLETTER
Stuff happens, basically and that means that CO2 can be stored at roughly the same density it would be as a liquid but without having to spend the energy to make it into one.
That means storing all of it on the back of a truck is a hell of a lot easier. So, that's cool but there are still energy demands and packaging issues for the system. To mitigate some of the former, the project proposes using yet another system, which is essentially a fancy fluid pump system that's like a heat pump but different, using the organic rankine cycle to capture energy from exhaust heat.
Phew, someone stick one on a GT car and argue about it for a full decade of regulations or something. Anyway, so: here you have it, fancy chemistry being used in a way that means CO2 can actually potentially be captured and stored on what's a normal heavy duty truck. If this system could be rolled out, it could save thousands of tonnes of CO2 annually.
For some scale of how pollutant heavy duty trucks are, I did some back-of-ciggy-packet maths about how much CO2 Mercedes F1 team might save with their switch to HVO100 in their haulage fleet for the European rounds of the Formula 1 season. Just from the 4% reduction at the tailpipe and with only 30 trucks over 15,000 miles, it'd be around 23.5t CO2 saving.
This system has the potential to reduce CO2 emissions by as much as 95%. So that'd be 558t CO2 saved under the same circumstances. (or 535.8t if the trucks were using HVO100 already)
Obviously, the ideal situation is not to emit CO2 in the first place. Like, just don't be burning that. However, logistics is intensely reliant on haulage and we have yet to detangle the vast routes that trucks follow, so developing viable systems that could abate it isn't just whataboutism or a distraction.
I meant to get into this here but I've definitely waffled on long enough already, so if you simply can't get enough of flue gases there's another study from Joule about catalysing exhaust fumes from industrial processes into a fuel gas and glycolic acid, as a method of simultaneously recycling plastics. Which is an interesting idea I probably need to dig further into.
Anyway, would you believe it. Car Science. On a Wednesday! Maybe people can change. Maybe I will finally finish the metamaterials one for Friday.
Hazel
x
:・゚✧:・.☽˚。・゚✧:・.:
Tips jar if you enjoyed this newsletter or wanna throw IndyCat some treats or whatever.
If you'd like to help non-financially, sharing this newsletter is really helpful to me! Buttondown doesn't have discoverability; I use it because I don't want to promote Substack, a platform that hosts transphobes and racists but it does come with some downsides, which is that it's not got the prominence of some other newsletter sites. So I'm always very happy for people to forward emails or share the web links to anyone who might be interested!
Honestly it sort of blows my mind that several hundred people subscribe to this already but it's super useful for me, in growing it, to have more. So if you do know anyone who might be interested and fancy sharing it, I super appreciate it.