Episode 2 - Eating moulds
Welcome to episode 2 of Filaments!
What if you could make a nutritious, protein-rich, meat-resembling product, in a matter of hours or days, with a fraction of the resources required to grow a cow? Let’s see how growing fungi could be a way for more efficient and possibly more sustainable food production. (Disclaimer: In 2019 and 2020 I was CTO of Mycorena, a start-up that works in exactly this area.)
EPISODE 2 - Eating moulds.
The story of Quorn and the future of food production
We eat mould all the time. Whether it’s considered yummy or yucky comes down to one question - are we expecting it to be there?
Unexpected: Mould on jam (accompanied by the age-old discussion of whether you should throw out the whole jar or just scoop out the fluffy bits). The cucumber at the bottom of the fridge, dissolving into a soft mess. The lemons, bought with the intention to use them in some dish you found on the internet two weeks ago, covered with grey-green dust.
Expected: Blue-mould cheese from the expensive cheese store in town. The fluffy white top of a Camembert. Cakes of perfectly formed, dense tempeh ready to be marinated and fried.

Tempeh, originally a fermented Indonesian food product made with legumes bound together by fungal mycelium. Credit: Silvia Hüttner.
Beer, cheese, bread, soy sauce… All of the intentional uses of fungi in food production once started as accidental discoveries, with spores delivered by wind, hands, grains and fruits. These discoveries happened centuries, or even millennia, ago. But there is one recent example where the use of a fungus to produce a new food product was actually the result of rational planning and careful screening of suitable strains. This lab-based approach resulted in what was called by its inventor “the first new food since the potato”.
Turning starch into protein
In the middle of the 20th century, many scientists as well as UN agencies warned that the world would soon run out of protein. Fears that animal products alone could not feed Earth’s growing population, and would cause famines and malnourishment, spurred research into alternative sources. It wasn’t long before food scientists started to turn to microorganisms for nutrition. Fungi and bacteria have the advantage of growing very efficiently under simple conditions. Most of them need just sugar, nitrogen, phosphorus and some minerals to grow and grow and grow and grow, doubling in number in as little as half an hour. Inside their cells they turn the nutrients they’ve taken up into edible protein, much more efficiently than any cow or chicken could ever do. This seemed like a perfect way to produce protein at a large scale.

Animation of bacteria growing and dividing. Credit: Giphy
As a large cereal producer, British food conglomerate Rank Hovis McDougall (RHM) had a lot of starch left over and started looking into ways to turn this abundant carbohydrate-rich byproduct into more sought-after protein. They collected over 3000 samples of filamentous fungi from all over the world to identify a strain that was safe to eat, would have a pleasant flavour and texture, and grew well on starch or sugar under laboratory and industrial conditions. The almost-too-good-to-be true story is, that despite combing the entire planet, the researchers eventually struck gold in 1967 in a field just a stone’s throw away from the RHM Technology Research Centre in Buckinghamshire, UK. The soil fungus they found, Fusarium venenatum, subsequently became the now hundred-million-dollar brand Quorn.
Turning a soil fungus into a new food product took Quorn many years of research, feeding trials in 12 animal species, and food safety assessments that included a two million word report on toxicology to get approval from the authorities. It also took time and money to develop and optimise the necessary large-scale production equipment to grow the fungus and turn it into food. But in 1985, this work was finally complete and Quorn launched on British supermarket shelves bearing the name “mycoprotein” (from myco = Greek for fungus) in the form of mince, “chicken” filets, sausages, and more. In the 35 years since, Quorn has gradually expanded into other countries. Occasionally, the brand faced some resistance on introduction, such as in the US where they were accused of mislabelling the products as “mushroom-based” (they now mention “mould” on their labels, as well as possible cross-reactions from people allergic to mould). Today Quorn is available in 14 countries worldwide.

"Chicken" filet from Quorn UK's recipe website. Credit: Quorn
How is mycoprotein made?
Mycoprotein, like Quorn, is made in large, up to 40-metre tall, stainless steel tanks (called bioreactors) that keep a mixture of sugar, water and some minerals at a certain temperature. Air is bubbled through the liquid, mixing it and saturating it with oxygen. Into this nutrient jacuzzi, a small volume of fungi starter culture is added, in a sterile way to make sure that nothing else but the specific fungal strain grows inside the tank. The fungal cells, surrounded by the most ideal conditions they could hope for, happily start to grow and divide, soon forming thick, fluffy clumps of mycelium that have a consistency somewhere between applesauce and wet cotton. If no more nutrients are added, the mycelium grows until all available sugar in the liquid is consumed and the whole process is completed in less than 24h.
The mycelium, aka the mycoprotein, can then be harvested by separating it from the liquid. The doughy mass of beige mycoprotein coming out of the bioreactor is surprisingly meat-like, since the fine, intertwined filaments of the mycelium resemble muscle fibres and give the product a chewy texture. Mycoprotein is high in protein and fibre content, and very low in fat and sugar. And what does it taste like? If you expect an aromatic mushroom-like taste you might be disappointed to learn that it doesn’t really taste of anything much. On the other hand, if you’re a food company looking for an easy meat replacement, you’re probably thrilled by this fact.
Quorn runs a continuous process, where grown mycelium is removed from the tank and fresh sugar-water is added at all times, keeping a steady stream of nutrients and space for new mycelium to grow [1]. In this way, a single process can run for up to six weeks churning out hundreds of kilos of mycoprotein per hour. Compare that to the time it takes for a cow to grow from conception to slaughterhouse, and it’s clear to see why mycoprotein production is such an incredibly efficient way to produce protein.
Side note: This way of growing microorganisms is either referred to as cultivation or fermentation. In a biochemistry-strict sense, fermentation only happens in the absence of oxygen, though the term has become somewhat looser in food production.
Finnish mycoprotein
Around the same time that Quorn was created in the UK, and prompted by the same motivations to increase worldwide protein production, a similar process was developed further North, with a very different outcome.
Finland’s forest cover is more extensive than that of any other European country, about 76% of the land is covered with trees. Naturally, pulp and paper production has been a very important industry for centuries, and the pulp mills dotting the landscape churn out not only paper but also large amounts of waste streams containing water, acids, other chemicals, and cellulose. As you can witness in a forest, many fungi can grow on wood and consume the sugars in the wood cellulose as food. Therefore, researchers in the 1960s looked into the possibility of using the cellulose-containing waste streams in paper mills as a nutrient source for growing fungi. Hundreds of strains were tested and one was identified as suitable to grow on these waste streams and produce high amounts of protein-rich mycelium.
The process was named Pekilo process, after the fungus they used, Paecilomyces variotii, and industrial production at two sites in Finland was established. Thousands of tonnes of mycoprotein were produced to be used initially as pig and chicken feed. Researchers also looked into using Pekilo as an ingredient in sausages, meatballs [2] and bread [3]. However, after only a decade, production of Pekilo protein was stopped and it never made it onto supermarket shelves. Why? New developments in the paper pulping process had led to changes in the waste stream composition, and at the same time interest in Pekilo protein decreased. The last Pekilo plant in Mänttä closed in 1992.
Quorn’s competitors
By the 1980s, fears of the “World Protein Gap” had faded and been debunked. In the 90s, Pekilo research was put into a filing cupboard, and Quorn - though successful - remained a niche product for vegetarians for most of the late 20th and early 21st century. All the while humanity continued to increase its taste for animal protein and found ever more ways to erode ecosystems and animal welfare through factory farming and feed plantations.
In recent years, however, due to greater awareness of the negative impact of meat production on the environment and meat consumption on personal health, sales of plant-based alternatives have grown enormously. Virtually every large food producer has soy, pea or gluten protein products on the market today. But when it comes to mycoprotein, Quorn is still the only option on supermarket shelves.
There are several possible reasons why other companies are still hesitant to make food from mycelium. The first is lack of expertise, as fungi cultivation and mycoprotein production require a very specific set of skills not traditionally found in the food industry. Another is concern that the association with mould could be a turn-off for consumers. There can also be considerable regulatory hurdles when it comes to using new ingredients in food - remember, it took Quorn a long time and a lot of money to get their mycoprotein approved. Finally, there’s the simple calculation that investing in very expensive and specialised equipment may just not be worth it when new alternative-meat products created with known ingredients (soy etc.) are performing so well.
Things are beginning to change, however. The need for greener food production is more pressing than ever, consumers are starting to crave new products (and are often willing to pay a higher price for them), and the boom in microbreweries has shown that it’s feasible to start successful fermentation businesses even if you’re not a multinational corporation. Not unimportantly as well, many of Quorn’s initial patents recently expired, making it possible to “copy” their process, and you can even order the Quorn strain from a culture collection.
Several small companies have popped up in the last couple of years that promise an updated, 21st-century version of how mycoprotein can help feed (and save) the world. They mostly focus on the prospective immense savings of water, land area, time and greenhouse gas emissions that can be achieved when comparing the production of a kilo of mycoprotein to a kilo of meat, as well as the advantages of mycoprotein over plant-based alternatives. Mycoprotein has a better nutritional profile than many plant proteins, offers a better (meaning in this case more meat-like) texture and can be grown faster on less land than most plants.
To differentiate themselves from Quorn, these companies innovate on different aspects of the production process. Some have developed new ways of growing fungi and cultivate the mycelium not submerged in liquid but instead in thick fluffy layers, that already resemble a meat-like structure. Others have spent a lot of effort on finding new fungal strains with improved taste or nutritional composition. Some companies focus on delivering a versatile B2B ingredient to other food companies, while others create and perfect a very specific mycoprotein end-product (e.g. “chicken”). There are also companies that attempt to make use of byproducts and side-streams from industry to feed the fungi, or even develop the old pulp & paper Pekilo process further.
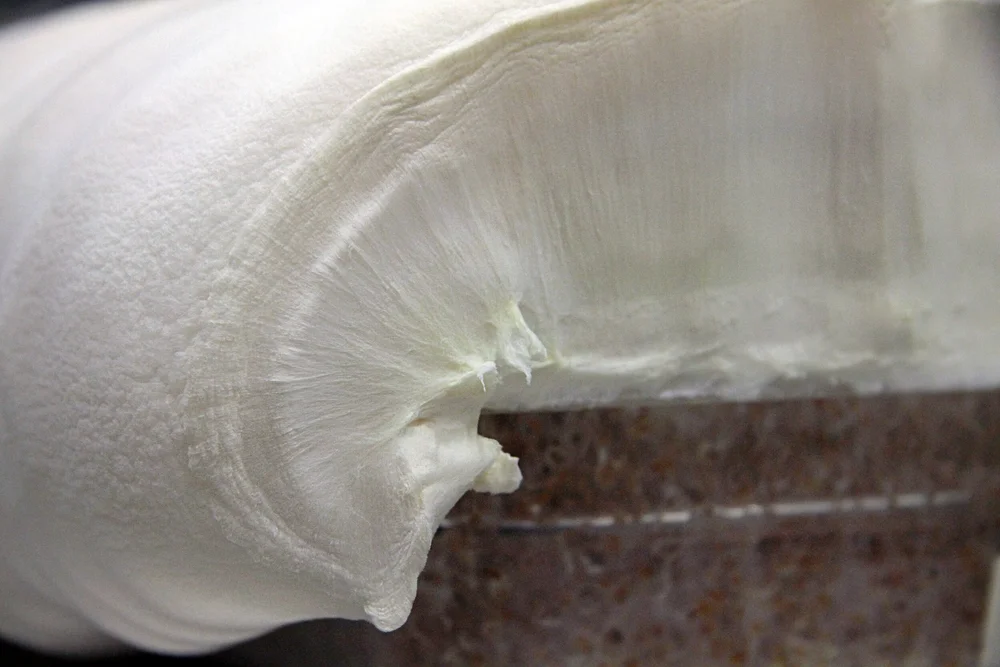
A layer of fluffy, edible mycelium ready to be transformed into “bacon”. Credit: Atlast Food
The future of food production
Growing food in a few days, in an area smaller than your kitchen table, sounds like the stuff of sci-fi novels, but we’re almost there. Tanks for mycoprotein production can be made in any size, and with some clever engineering, personal myco-fermenters could soon be a normal appliance in our homes. Or, if not in our homes, then a decentralised food production system could be envisioned, where villages or communities might manufacture their own mycoprotein. Similarly, mycoprotein production could happen at many different sites in connection with other industries that produce suitable side-streams which are then directly fed into the fungi cultivation tanks, as pioneered by the Pekilo process.
Since the fungal growth process happens in a controlled, sealed-off environment, this type of food production is largely independent of weather, climate or soil conditions and can therefore happen in places where normal agriculture is impossible (and increasingly more impossible due to climate change). Of course, the need for clean water and electricity for the fermentation present a challenge, but one that will hopefully be solved in the near future by improved production processes (e.g. water recycling) and better availability of solar and wind power. Currently, the carbon footprint of mycoprotein is still slightly worse than that of plant proteins or even chicken [4], mostly because of the high electricity demand of heating, cooling, stirring and aerating the bioreactors. And while chicken farming is certainly not great for chickens, its greenhouse gas emissions are actually relatively low compared to other meats (such as beef). But mycoprotein production is already a clear winner in some aspects, such as water pollution (no manure runoff into waterways!) or land use.
What about lab-grown meat? In the last few years it has attracted a lot of attention and investment and has, like mycoprotein, been touted as a “game changer” for more sustainable food production. Unfortunately, the animal cells that are used for lab-grown meat are more delicate and fussy than fungi cells, grow slower and require more complicated bioreactor setups and conditions (e.g. scaffolds, vitamins, growth factors). Lab-grown meat has still not been produced at large scale, while mycoprotein is actually a comparatively mature technology. At the time of writing, it’s questionable if lab-grown meat will ever live up to its promises of being a more sustainable meat alternative [5].
Either way, for some people, growing our food in temperature-controlled tanks might be a step too futuristic or “unnatural”. But if it means drastically reducing the environmental impact, not slaughtering billions of animals on a daily basis and being able to produce tasty and nutritious food in a reliable manner, it’s perhaps worth revisiting those reservations.
In the next episode: Fungi as materials. Wearing fungi, living in fungi, being buried in fungi.
[1] 21st Century Guidbook to Fungi. Moore et al., 2019. http://www.davidmoore.org.uk/21st_Century_Guidebook_to_Fungi_PLATINUM/Ch17_18.htm
[2] Uses of Pekilo, a microfungus biomass from Paecilomyces varioti in sausage and meat balls. Koivurinta et al., 1979. https://ifst.onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2621.1979.tb00902.x
[3] Bread baking properties of Pekilo, a microfungus biomass from Paecilomyces varioti. Koivurinta et al., 1980. https://onlinelibrary.wiley.com/doi/abs/10.1002/food.19800240702
[4] Meat alternatives: life cycle assessment of most known meat substitutes. Smetana et al., 2015. https://doi.org/10.1007/s11367-015-0931-6
[5] Climate Impacts of Cultured Meat and Beef Cattle. Lynch & Pierrehumbert, 2019. https://doi.org/10.3389/fsufs.2019.00005
Thank you for subscribing to this limited run newsletter about the fascinating things fungi can do and how we can use them. My name is Silvia Hüttner, I’m a biotechnology PhD, fungi enthusiast and a researcher specialised in fermentation and enzyme technology. I’d love to hear from you if you have any questions, comments or suggestions, or just want to say hi. 🍄